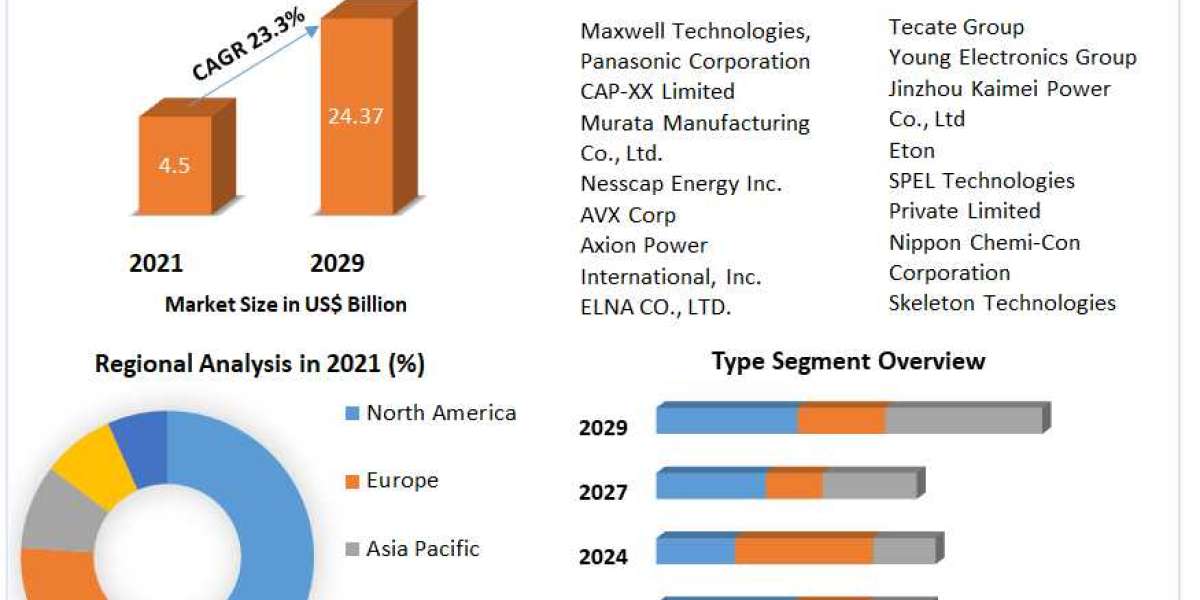
Pharmaceutical Contract Development and Manufacturing Organizations (CDMOs) Market are companies that provide a range of services to pharmaceutical and biotech companies, including drug development, formulation, manufacturing, and packaging.
CDMOs are often used by small and mid-sized pharmaceutical companies that lack the resources or expertise to develop and manufacture drugs in-house. By outsourcing these activities to a CDMO, these companies can accelerate their drug development timelines and reduce costs.
CDMOs offer a range of services, including:
- Drug development and formulation: CDMOs can provide expertise in drug development and formulation, including preclinical and clinical development, analytical testing, and formulation optimization.
- Manufacturing: CDMOs can manufacture drugs in a variety of forms, including tablets, capsules, injectables, and topical formulations. They may also provide sterile manufacturing capabilities for injectable drugs.
- Packaging and labeling: CDMOs can provide packaging and labeling services for pharmaceutical products, including primary and secondary packaging, labeling design and printing, and serialization.
- Supply chain management: CDMOs can manage the entire drug supply chain, including raw materials sourcing, inventory management, and distribution.
CDMOs can offer a range of benefits to pharmaceutical companies, including access to specialized expertise, reduced development timelines, and cost savings through economies of scale. As a result, the CDMO market has grown rapidly in recent years, with many new entrants and mergers and acquisitions among existing players.
Market DynamicsTop of Form
- Europe Pharmaceutical CDMO Market
The pharmaceutical CDMO market has been growing rapidly in recent years and is expected to continue to grow in the coming years. Some of the key market dynamics driving this growth include:
- Increased outsourcing by pharmaceutical companies: Pharmaceutical companies are increasingly outsourcing drug development and manufacturing activities to CDMOs to reduce costs and accelerate development timelines. This trend is expected to continue as pharmaceutical companies face increasing pressure to bring new drugs to market quickly and cost-effectively.
- Growth in biologics: The growth of biologics, which are more complex and require specialized manufacturing processes, is driving demand for CDMOs with expertise in biologics manufacturing.
- Capacity constraints: As demand for CDMO services has grown, there have been concerns about capacity constraints in the industry. This has led to investments in new manufacturing facilities and capacity expansions by CDMOs.
- Increasing regulatory requirements: Regulatory requirements for drug development and manufacturing continue to become more complex, which is driving demand for CDMOs with expertise in navigating regulatory requirements.
- Mergers and acquisitions: The pharmaceutical CDMO industry has seen a significant amount of merger and acquisition activity in recent years, as companies seek to expand their capabilities and geographic reach.
Overall, the pharmaceutical CDMO market is expected to continue to grow in the coming years, driven by increasing outsourcing by pharmaceutical companies, growth in biologics, and increasing regulatory requirements. However, the industry will need to continue to invest in capacity expansion and expertise to meet growing demand.
Top of Form
COVID-19 Impact on Europe Pharmaceutical CDMO Market
The COVID-19 pandemic has had a significant impact on the pharmaceutical CDMO market. Some of the key impacts include:
- Delays in clinical trials: Clinical trials have been disrupted by the pandemic, leading to delays in drug development timelines. This has impacted the demand for CDMO services, as pharmaceutical companies have postponed outsourcing activities until clinical trials can resume.
- Increased demand for COVID-19-related products: The pandemic has also led to increased demand for products such as vaccines, antiviral drugs, and diagnostic tests. This has created new opportunities for CDMOs with expertise in these areas, but has also strained capacity in the industry.
- Supply chain disruptions: The pandemic has disrupted global supply chains, leading to shortages of raw materials and other inputs. This has impacted CDMOs, which rely on these inputs to manufacture drugs.
- Increased focus on drug safety: The pandemic has increased the focus on drug safety, particularly for vaccines and other COVID-19-related products. This has led to increased regulatory scrutiny and requirements, which can increase the complexity and cost of drug development and manufacturing.
- Shift towards virtual operations: The pandemic has accelerated the trend towards virtual operations, including virtual clinical trials, remote monitoring, and telemedicine. This has led to new opportunities for CDMOs with expertise in virtual operations, but has also created new challenges in terms of data management and cybersecurity.
Overall, the COVID-19 pandemic has had a mixed impact on the pharmaceutical CDMO market. While it has created new opportunities for some companies, it has also disrupted drug development timelines, strained capacity, and increased regulatory requirements. As the pandemic continues to evolve, the full impact on the industry will continue to be monitored.
Get Free Sample Research Copy for More Industry Insights:
https://www.axiommrc.com/request-for-sample/11200-europe-pharmaceutical-cdmo-market-report
Europe Pharmaceutical CDMO Market Segmental Overview
The study analysed the Europe Pharmaceutical CDMO market based on service type, research phase and geography
Europe Pharmaceutical CDMO Market by Service Type
Pharmaceutical Contract Development and Manufacturing Organizations (CDMOs) offer a wide range of services to their clients in the pharmaceutical and biotech industries. Some of the key service types offered by CDMOs include:
- Drug development and formulation: CDMOs can provide expertise in drug development and formulation, including preclinical and clinical development, analytical testing, and formulation optimization.
- Manufacturing: CDMOs can manufacture drugs in a variety of forms, including tablets, capsules, injectables, and topical formulations. They may also provide sterile manufacturing capabilities for injectable drugs.
- Packaging and labeling: CDMOs can provide packaging and labeling services for pharmaceutical products, including primary and secondary packaging, labeling design and printing, and serialization.
- Supply chain management: CDMOs can manage the entire drug supply chain, including raw materials sourcing, inventory management, and distribution.
- Analytical and testing services: CDMOs can provide a range of analytical and testing services to their clients, including method development and validation, stability testing, and quality control testing.
- Regulatory support: CDMOs can provide regulatory support to their clients, including assistance with regulatory filings, inspections, and audits.
- Technology transfer: CDMOs can help their clients transfer manufacturing processes from one location to another, including process optimization and validation.
- Clinical trial services: Some CDMOs offer clinical trial services, including patient recruitment, data management, and statistical analysis.
Overall, CDMOs offer a wide range of services to their clients, from drug development and formulation to supply chain management and regulatory support. By outsourcing these activities to a CDMO, pharmaceutical and biotech companies can accelerate drug development timelines, reduce costs, and access specialized expertise.
Europe Pharmaceutical CDMO Market by Research PhaseTop of Form
The European pharmaceutical CDMO market by research phase can be segmented as follows:
- Preclinical Services: CDMOs offer preclinical services to pharmaceutical and biotech companies for the testing of their drug candidates. These services may include in vitro and in vivo studies, pharmacokinetic and toxicology studies, and formulation development.
- Phase I Services: CDMOs provide services for Phase I clinical trials, including manufacturing of clinical trial materials, formulation development, analytical testing, and regulatory support.
- Phase II Services: CDMOs offer Phase II services, including scale-up and manufacturing of clinical trial materials, process optimization, analytical testing, and regulatory support.
- Phase III Services: CDMOs provide Phase III services, including large-scale manufacturing of drug candidates for clinical trials, process validation, and regulatory support.
- Commercial Manufacturing Services: CDMOs also offer commercial manufacturing services to pharmaceutical and biotech companies for the large-scale production of approved drugs. These services may include process optimization, technology transfer, analytical testing, and regulatory support.
The European pharmaceutical CDMO market by research phase is influenced by factors such as the increasing demand for outsourcing services, the growing prevalence of chronic diseases, and the need for cost-effective drug development and manufacturing. As a result, CDMOs are expected to continue to play an important role in the pharmaceutical industry, providing specialized expertise and services across all stages of the drug development process.
Europe Pharmaceutical CDMO Market by CountryTop of Form
The European pharmaceutical CDMO market by country can be segmented as follows:
- Germany: Germany is the largest pharmaceutical market in Europe, with a well-developed pharmaceutical industry and a strong focus on research and development. The country is home to several major CDMOs and is expected to continue to be a key market for CDMO services in the coming years.
- United Kingdom: The United Kingdom is a major player in the European pharmaceutical industry, with a significant focus on research and development. The country is home to several major CDMOs and is expected to continue to be an important market for CDMO services post-Brexit.
- France: France has a well-developed pharmaceutical industry and is home to several major pharmaceutical companies. The country is expected to be an important market for CDMO services in the coming years.
- Italy: Italy has a growing pharmaceutical industry, with a focus on research and development in areas such as oncology and rare diseases. The country is expected to be an important market for CDMO services as the pharmaceutical industry continues to grow.
- Spain: Spain has a growing pharmaceutical industry and is home to several major pharmaceutical companies. The country is expected to be an important market for CDMO services in the coming years.
Other countries in Europe, such as Switzerland, Belgium, and the Netherlands, also have well-developed pharmaceutical industries and are expected to be important markets for CDMO services. The European pharmaceutical CDMO market by country is influenced by factors such as government regulations, investment in research and development, and the availability of skilled labor.
Europe Pharmaceutical CDMO Market Key Players
The key competitor of the market includes Almac Group Ltd., B. Braun Melsungen AG, Boehringer Ingelheim International GmbH, Jubilant Life Sciences Ltd., Lonza Group Ltd., Recipharm AB
Buy Now and Get More Discount:
https://www.axiommrc.com/buy_now/11200-europe-pharmaceutical-cdmo-market-report
About Us
Axiom Market Research Consulting™ is a full-service market research and data analytics company providing key market intelligence to global companies to take informed business decisions pertaining to their marketing strategy, investments, new product launches, market competition, consumer or end users, social media trends etc.
Axiom Market Research Consulting™ offers market research services such as syndicated market research, custom market research, business consulting, and consumer/end user surveys. Under Business to Consumer (B2C) market research offerings, Axiom MRC assists its clients in finding quantitative information/preferences of its brands and services such as, awareness, usages, satisfaction, tracking, ethnicity etc. Axiom MRC offers data collection services through online surveys, social media, data processing and interpretation.
Axiom MRC with its experienced team of research and data analysts, has delivered more than 5000+ Market Research Projects, 3800+ Data Analytics Projects, 1200+ Business Support Projects and has a 800+ Global Client Base. Axiom Market Research Consulting™ aims to become the preferred market research and data analytics company by providing key market intelligence solutions for client’s business growth.
Contact Us:
Axiom Market Research Consulting™
3 Germay Dr. Ste 4 - 4666
Wilmington DE 19804
U.S.:- + 1 (845) 875-9786
U.K.:- + 44 (0) 20 3286 9707
Email: [email protected]
Website: https://www.axiommrc.com/
http://contactsnleads.com/
Blog: https://industrywatch24.com/
https://readbeyondnews.com/
Follow On
LinkedIn: https://www.linkedin.com/company/axiom-market-research-and-consulting/
Twitter: https://twitter.com/AxiommrcCom
Instagram: https://www.instagram.com/axiom_mrc
Facebook: https://www.facebook.com/axiommrc
Top of Form