The global In Vitro Diagnostics and IVD Quality Control Industry was estimated at USD 112.79 Billion in 2021 and is anticipated to increase at a significant CAGR of 0.2% from 2022 to 2030.
Grand View Research’s In Vitro Diagnostics and IVD quality control industry data book is a collection of market sizing & forecasts insights, regulatory & technology framework, pricing intelligence, volumetric analyses, competitive benchmarking analyses, macro-environmental analyses studies. Within the purview of the database, such information is systematically analyzed and provided in the form of summary presentations and detailed outlook reports on individual areas of research.
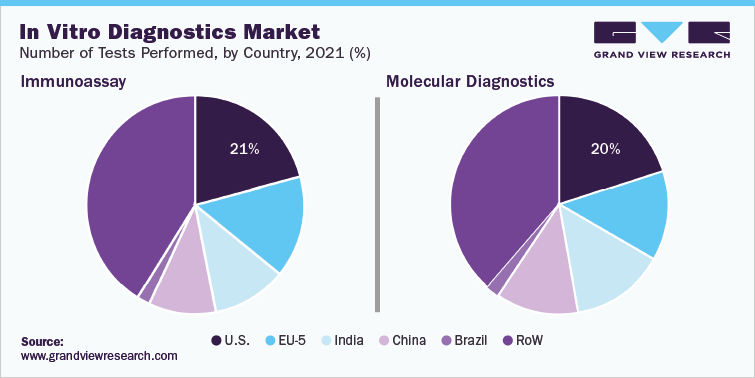
In Vitro Diagnostics Market Insights
The global in vitro diagnostics (IVD) market size was estimated at USD 111.67 Billion in 2021 and is projected to grow at a compound annual growth rate (CAGR) of 0.2% from 2022 to 2030. The growth can be attributed to increasing adoption of IVD owing to a rise in the incidence of infectious and chronic diseases. The development of automated IVD systems for laboratories and hospitals to provide efficient, accurate, and error-free diagnoses is expected to fuel market growth. The rising number of IVD products being launched by key players is also fueling market growth. For instance, in November 2023, ARUP Laboratories received a CE mark from EU-IVDR for AAV5 DetectCDx, a companion diagnostic to select the eligibility of severe hemophilia A-affected patients for BioMarin’s new gene therapy, Roctavian.
Favorable initiatives undertaken by government and non-government bodies to improve overall healthcare services are anticipated to increase market growth. In October 2023, the WHO published the Essential Diagnostics List (EDL), a comprehensive list of IVD products that helps countries make decisions regarding diagnostic tools. It provides evidence-based recommendations and ensures the accessibility of essential products for target people. Moreover, in August 2023, the Africa CDC collaborated with the Africa Development Agency-New Partnership for Africa's Development (AUDA-NEPAD) to increase access to diagnostic tests across Africa. Such initiatives are expected to boost market growth.
A rise in the geriatric population and growth in knowledge regarding early testing have led to a surge in the number of regular check-ups, as a majority of deaths due to infections and chronic conditions occur in the population aged over 75 years. As per the Office for Budget Responsibility, UK, healthcare costs have risen exponentially, which can create economic pressure on nations with rapidly growing geriatric population. However, this expenditure is anticipated to translate positively for the IVD industry, driving market growth.
Order your copy of the Free Sample of “In Vitro Diagnostics And IVD Quality Control Industry Data Book - In Vitro Diagnostics & IVD Quality Control Market Size, Share, Trends Analysis, And Segment Forecasts, 2022 - 2030” Data Book, published by Grand View Research
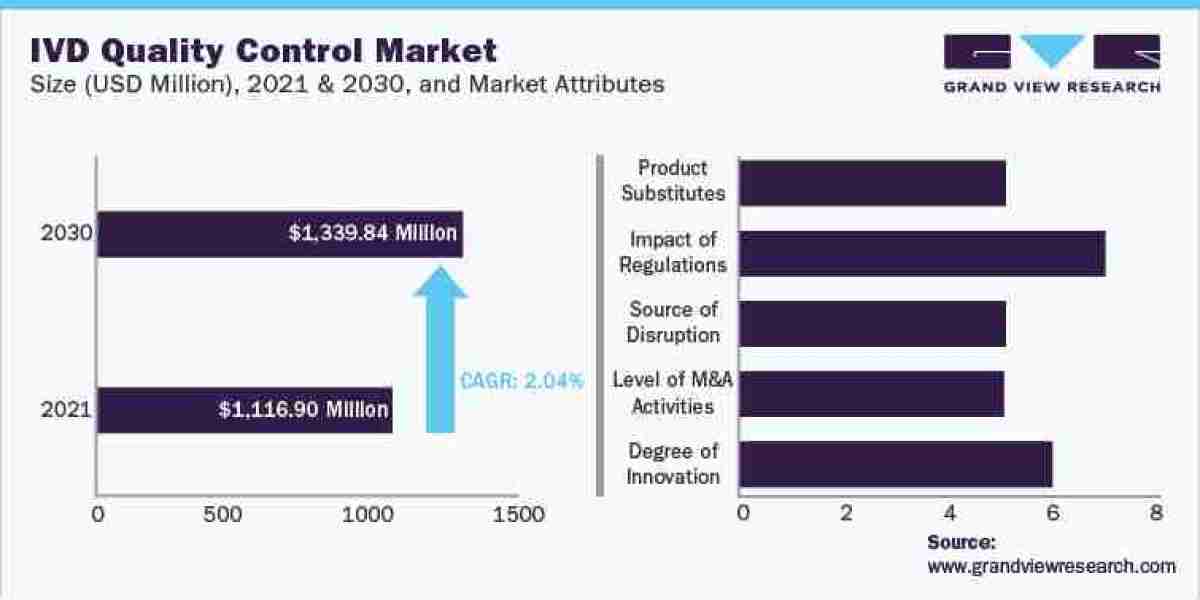
IVD Quality Control Market Insights
The global in vitro diagnostics quality control market size was estimated at USD 1.12 Billion in 2021 and is expected to grow at a compound annual growth rate (CAGR) of 2.0% from 2022 to 2030. An increasing number of accredited clinical laboratories worldwide and the presence of favorable regulatory bodies are expected to be the key factors driving the market growth. Due to the high prevalence rate of diseases, such as diabetes, cardiovascular diseases, and infectious diseases, diagnostic laboratories have gained demand. Many private as well as public laboratories are undergoing laboratory accreditation procedures to meet industry standards, improve their procedural volume, and attract more patients. Laboratories accredited by CLIA are eligible for reimbursement through Medicare and Medicaid Services.
The regulatory bodies monitoring quality control and regular quality check of in vitro diagnostics (IVD) devices and service providers include FDA for U.S.; Medicines and Healthcare Products Regulatory Agency (MHRA) for the UK; Therapeutic Goods Administration (TGA) for Australia; Central Drug Standard Control Organization (CDSCO) for India; Health Canada for Canada; European Medicines Agency (EMEA) for Europe; Ministry of Health, Labor & Welfare(MHLW) for Japan; Ministry of Health, Labor & Welfare (MHLW) for Brazil; and Ministry of Health for South Africa.
Moreover, Global Harmonization Task Force (GHTF) regulatory authorities, such as the U.S. FDA, are encouraging convergence of regulatory systems for medical devices, which is anticipated to encourage trade, while protecting public health through regulatory means. The American Clinical Laboratory Association states that more than 7.5 billion lab tests are performed in the U.S. annually and 80% of clinical decisions are taken after laboratory testing. According to the WHO, an estimated 537 million adults aged 20–79 years are suffering from diabetes. This represented 10.5% of the world’s population in this age group in 2021.
Go through the table of content of In Vitro Diagnostics and IVD Quality Control Industry Data Book to get a better understanding of the Coverage & Scope of the study
In Vitro Diagnostics and IVD Quality Control Industry Data Book Competitive Landscape
Companies are focusing on expansions, development of innovative medical devices, and technological advances. Moreover, mergers and acquisitions for novel product development constitute some of the other strategic initiatives implemented by key players.
Key players operating in the In Vitro Diagnostics and IVD Quality Control Industry are:
- Una Health Ltd.
- Diachel Diagnostics S.A.
- BHR Diagnostics Pvt. Ltd.
- MD Doctors Direct GmbH
- Axon Lab AG
Check out more Industry Data Books, published by Grand View Research
About Grand View Research
Grand View Research, U.S.-based market research and consulting company, provides syndicated as well as customized research reports and consulting services. Registered in California and headquartered in San Francisco, the company comprises over 425 analysts and consultants, adding more than 1200 market research reports to its vast database each year. These reports offer in-depth analysis on 46 industries across 25 major countries worldwide. With the help of an interactive market intelligence platform, Grand View Research helps Fortune 500 companies and renowned academic institutes understand the global and regional business environment and gauge the opportunities that lie ahead.
Contact:
Sherry James
Corporate Sales Specialist, USA
Grand View Research, Inc.
Phone: 1-415-349-0058
Toll Free: 1-888-202-9519
Email: [email protected]
Web: https://www.grandviewresearch.com/sector-reports-list
Follow Us: LinkedIn | Twitter