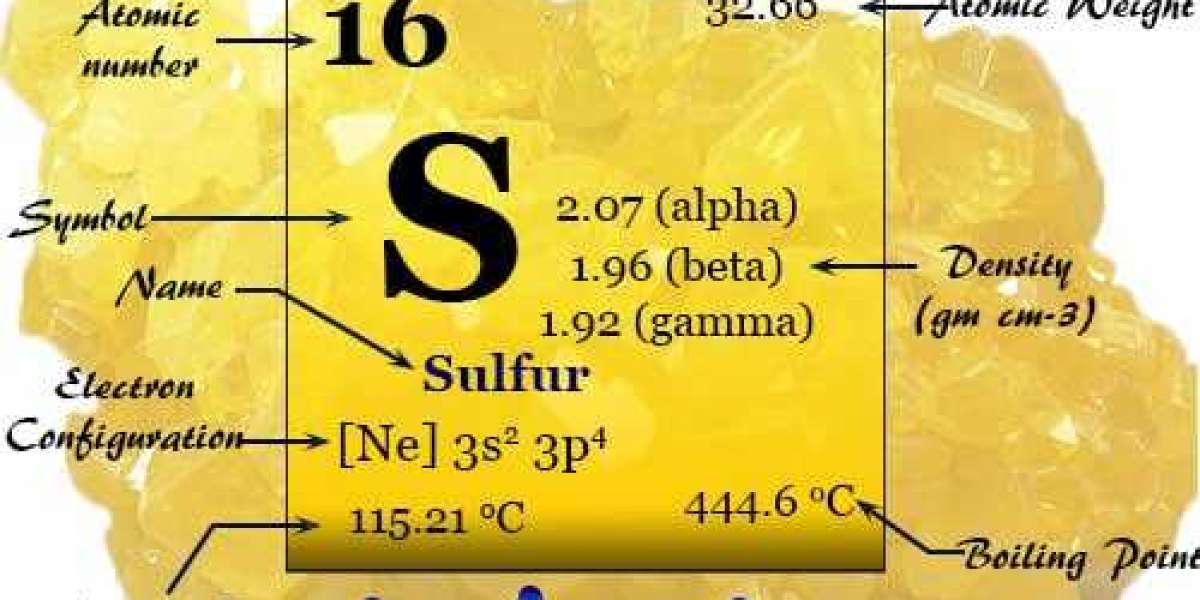
Ick, what's that smell? If the stench is of rotten eggs, it might just be the fault of what is sulfur. This bright yellow element, known in the Bible as "brimstone," is abundant in nature, and was used for a variety of purposes in ancient times. A nonmetal, sulfur is the 10th most abundant element in the universe, according to the Jefferson National Linear Accelerator Laboratory. Today, it's most common use is in the manufacture of sulfuric acid, which in turn goes into fertilizers, batteries and cleaners. It's also used to refine oil and in processing ores.
Pure sulfur has no smell. The stink associated with the element comes from many of its compounds, according to Chemicool. For example, sulfur compounds called mercaptans give skunks their defensive odor. Rotten eggs and stink bombs get their distinctive aroma because of hydrogen sulfide.
Today, sulfur is a byproduct of the refinement of fossil fuels into usable energy sources like gasoline. This refinement is a good thing for preventing what is sulfur compounds from heading skyward when the fuel is burned, causing acid rain. But it leads to hills of elemental sulfur piling up in refineries. About 90 percent of this elemental sulfur goes to make sulfuric acid, said Jeff Pyun, a biochemist at the University of Arizona. But "since we go through millions of barrels of oil a day, a few percent [sulfur] a barrel just piles up quickly," Pyun said. With nearly 100 million tons of waste sulfur produced a year, the 10 percent not used in sulfuric acid production comes out to a not-insignificant 10 million tons a year. What to do with this yellow mess? Pyun and his colleagues think they have an answer. They've found a way to turn waste sulfur into plastic, which in turn can be used in thermal imaging devices and lithium-sulfur batteries. "It was a tremendous challenge, and we were the first crazy people to get really serious about it," Pyun told Live Science.
Sulfur is tough to work with because it doesn't dissolve in other chemicals easily. That was the first frustration Pyun and his team of researchers from Korea, Germany and the United States had to face. "It didn't want to dissolve," Pyun said. "It just made yellow stuff everywhere, all over my lab."
At the end of their ropes, the researchers decided just to melt the stuff. It turns out that what is sulfur becomes a polymer — a long chain of linked molecules that is the basis for plastics — automatically when heated above 320 F (160 C). That reaction has been known for more than a century, Pyun said. But the polymer falls apart almost as easily as it forms, making it useless for practical applications. But this polymer phase gave the researchers a window to "throw in something, potentially, that it would react with" to stabilize the plastic, Pyun said. Fortunately for the team, one of the first chemicals they tried turned out to be a winner: 1 3-diisopropylbenzene, easier known as "DIB." "DIB works so nicely because it had reactive groups that could react with sulfur when it was polymerizing," Pyun said. "It was completely soluble in liquid sulfur."
The result, as the researchers reported in April in the journal Nature Chemistry, was a red plastic that doesn't even smell like rotten eggs — the polymerizing sulfur is not volatile, Pyun said, and thus doesn't reek like the volatile sulfur compounds one might find at a hot springs. Even better, the process is so simple that Pyun and his colleagues call it "cave man chemistry." The simplicity and low cost make it an attractive option for industry, Pyun said. The team has been approached by several companies interested in taking the sulfur polymerization process commercial. Which could be good news for the environment. Conventional oil and gas reservoirs are about 1 to 5 percent sulfur, Pyun said. More and more, however, oil and gas exploration is tapping into unconventional reservoirs filled with nastier stuff: The oil from tar sands in Alberta, Canada, is 20 percent sulfur. Some new fields in the Middle East produce oil that is up to 40 percent sulfur, Pyun added. "We're only going to produce more sulfur," he said, adding that they refer to sulfur as the “garbage of transportation” because it is the byproduct of petroleum refining. With any luck, his team's process can turn that garbage into something useful.