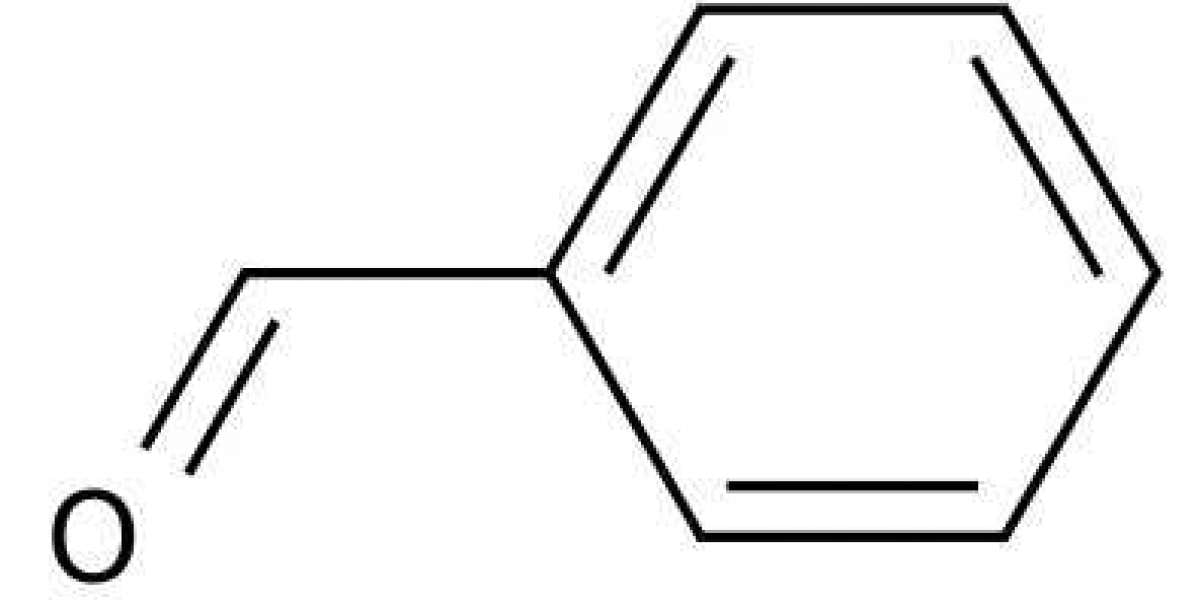
The simplest aromatic aldehyde, which consists of a benzene ring with a formyl (-CHO) substituent, is termed benzaldehyde (C6H5CHO). This organic chemical compound has several industrial applications, including dyes, flavouring agents, perfumes and the manufacturing of several other organic compounds. Found naturally in glycoside amygdalin, this aromatic aldehyde is known for its distinct, almond oil-like taste and odour. The molecular formula of this compound is C7H6O, and its IUPAC name is benzenecarbaldehyde. It is also referred to as several other names, including benzenedicarboxaldehyde, phenylmethanal, or benzoic aldehyde. It appears as a clear liquid with a smell like almonds. It is miscible with volatile oils, ether, and alcohol. It is denser than and thus only slightly soluble in water and has a solubility of 3 g/L.
Discovery of Benzaldehyde
Benzaldehyde occurs in a variety of natural items such as almonds or cherries. The isolation of benzaldehyde from bitter almonds goes back to 1803 and is credited to a French pharmacist Martrès. It was later studied by the German chemists Justus von Liebig and Friedrich W?hler, who first synthesised it successfully in the 1830s. This led to the establishment of the structural theory of organic chemistry.
Structure of Benzaldehyde
The structure of benzaldehyde consists of a benzene ring substituted with an aldehyde unit. This formyl unit has one atom of carbon, hydrogen, and oxygen. The number of benzaldehyde sigma bonds is 14, formed by the head-to-head overlapping of atomic orbitals.
Preparation of Benzaldehyde
This simple aromatic aldehyde can be derived from a variety of natural sources. It can also be manufactured synthetically from toluene which is found in crude oil, for industrial usage. This is done by a series of chemical reactions in which toluene reacts with chlorine. This leads to the formation of benzal chloride, which can be further treated with water to form benzaldehyde. Benzaldehyde can also be synthesised by the oxidation of benzyl alcohol and carbonylation of benzene. Alkaline hydrolysis of benzal chloride also yields benzaldehyde.
Uses of Benzaldehyde
Benzaldehyde is an aromatic compound with a distinct odour resembling almonds. It can be extracted from a variety of natural sources and can also be synthesised by liquid phase chlorination of toluene. There is no chemical distinction between these two types of benzaldehyde. Benzaldehyde is a widely used compound in the chemical industry. It also finds usage in several other items. The most common applications of benzaldehyde that make it such an important compound are listed below-
1.The most common use of benzaldehyde is to provide almond flavouring to items. It is used in food and beverages to provide the scent of almonds. Various scented products also use benzaldehyde as an additive for having a distinct odour.
2.The industrial usage of benzaldehyde is that it acts as an intermediate in the production of several organic compounds. It is a precursor to various chemical additives, plastics, and pharmaceutical products.
3.Another significant use of the odour of benzaldehyde is as a bee repellent. It is used to draw the bees away from a honeycomb in order to extract the honey from these structures.
4.Some cosmetic products and personal care products also contain benzaldehyde.
5.Benzaldehyde is also used in the production of dyes (acridine and aniline dyes), soaps, and perfumes.
6.It is also used in cakes and baked goods as almond extract. Benzaldehyde is also used in additives like antibacterial and antifungal preservatives.
7.It is an intermediary product in the formation of compounds like cinnamic acid, benzoic acid, etc.
8.It is used as a solvent for oils, resins, ethers, etc.
The flavouring and fragrance of benzaldehyde are the reason for its widespread use in several industries.
Storage of Benzaldehyde
Benzaldehyde is usually stable but should be stored as per the recommended conditions. It should be properly stored in a sealed container away from heat and light. It should be kept away from reactive substances such as acids and reducing agents. It is also recommended to store this compound under nitrogen.